Read our Blogs
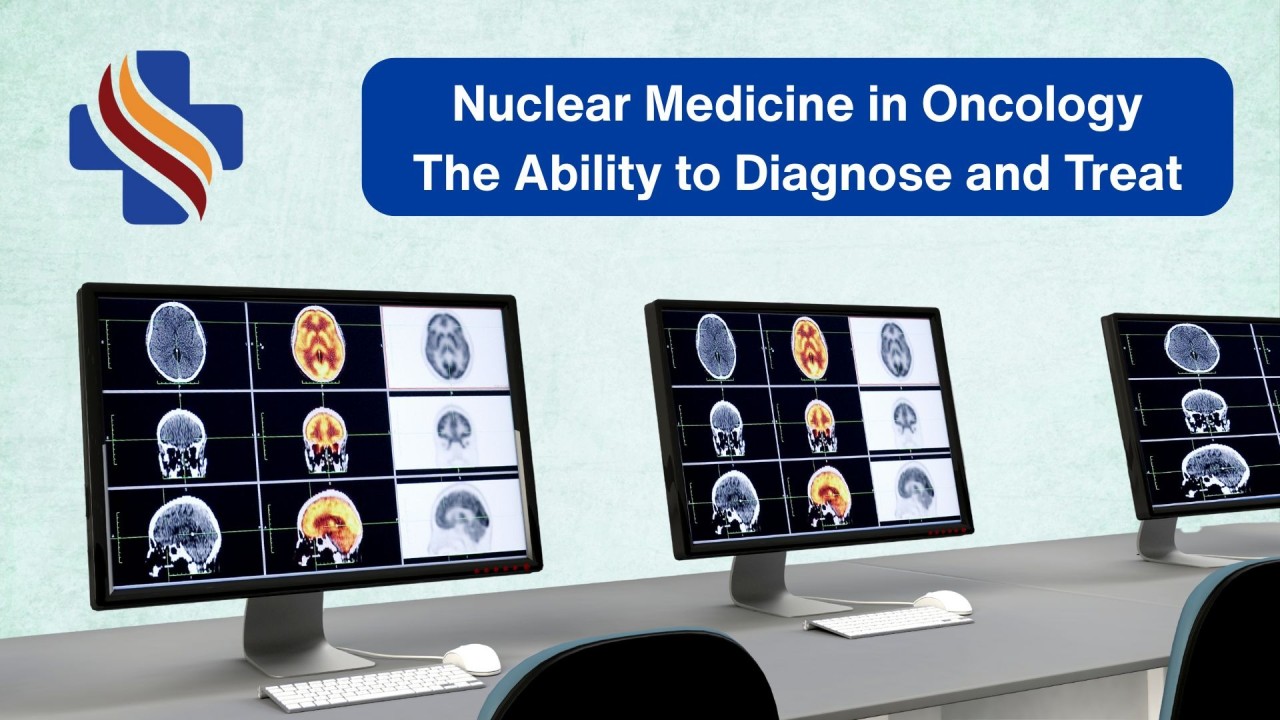
Nuclear Medicine in Oncology: Lighting the Way in Cancer Diagnosis & TherapyThe Story Begins with a Tracer
The foundation of nuclear medicine goes back to a groundbreaking idea from Hungarian chemist George de Hevesy in the early 20th century, a Nobel Prizewinning scientist and also widely recognized as the "father of nuclear medicine"
Hevesy discovered that minute quantities of radioactive compounds (radiopharmaceuticals) / radioactive tracers could be used to follow chemical processes inside living beingsjust like adding a drop of dye to water to see where it flows.
In his famous experiment, he used tiny amounts of radioactive isotopes to study how plants absorbed nutrients.
Thistracer principle is still the basis of nuclear medicine today: ➡️ Use a safe radioactive compound (called a radiotracer) that behaves like natural substances in the body. ➡️ Track its path with special cameras. ➡️ Learn how organs and diseases functionfrom the inside out.
<div class="ivm-view-attr__img-wrapper
">
The Fundamental Principle of Nuclear Medicine
What is Nuclear Medicine?
Nuclear medicine is a branch of medical imaging and therapy that uses small amounts of radioactive materials (called radiotracers) to diagnose and treat diseases, especially cancer. These tracers are injected, swallowed, or inhaled, and they target specific organs or tissues. Special cameras detect the radiation emitted, creating images that show how your body is functioning at a molecular levelnot just its structure.
Unlike X-rays or MRI that show body structures, nuclear medicine looks at how the body works at a cellular level - like how cells are metabolizing or if a tumor is active.
Small amounts ofradioactive tracers (safe, medical-grade) are used.
Special scanners detect these tracers and create detailed images.
The result? Doctors dont just seewhere something is, but also how active it is.
It has two main arms:1. Imaging using:
Conventional Nuclear Medicine (Gamma Camera / SPECT)
Molecular Imaging (PET-CT)
2. NM therapy or now popularly known as Theranostics (Diagnosis + Therapy with the same molecule)
Conventional Nuclear Medicine The Gamma Camera Era
Uses gamma cameras to detect radioactive tracers and Produce images of organ function.
Common tracers: 99mTc (Technetium-99m) based RPs most widely used isotope globallyThe workhorse tracer, used in over 80% of procedures. Used for bone scans, renal scans, cardiac scans, thyroid scans. Iodine I-123 / I-131: Benign and malignant thyroid diseases - Targets thyroid tissue. In diagnosis, it scans for thyroid cancer; in therapy, higher doses destroy cancerous cells.
Helps in cancer by: Detecting bone metastases (spread of cancer to bones). Assessing thyroid cancer.
PET-CT The Molecular Imaging Revolution
PET-CT combines positron emission tomography (PET) with CT.
Shows both function (PET) and structure (CT) in one scan.
Uses radiotracers that mimic natural body chemicals or target receptors in various physiological and patholigcal conditions.
Common PET tracers in cancer:
18F-FDG (Fluorodeoxyglucosewith F-18) Mimics sugar, detects sugar-hungry cancer cells and used in most of the cancers including lung, lymphoma, breast and colorectal cancers.
68Ga-PSMA prostate cancer.
68Ga-DOTATATE neuroendocrine tumors.
FES (Fluoroestradiol) breast cancer (estrogen receptor imaging).
Role in Cancer Diagnosis 👉PET-CT is often called the GPS for cancer, guiding doctors at every step:
Early detection: Using various metabolic and functional processes such as FDG PET scan targetting glucose metabolism: Cancer cells consume more energynuclear scans pick this up early.
Accurate staging: Shows if cancer has spread.
Treatment planning: Helps doctors decide surgery, chemotherapy, or radiation approaches.
Treatment Response Assessment: Shows if cancer is responding or not to treatment
Follow-up: Detects recurrence earlier than most tests.
Role in Cancer Therapy: 🎯 Theranostics Treat What You See and Diagnose and Treat in One Go
Nuclear medicine is not just about diagnosisit can also treat cancer.
Theranostics = Therapy + Diagnostics.
Uses the same molecule for both diagnosis and treatment, just with different isotopes, For e.g. 68GaPSMA for Prostate cancer staging using PET CT and 177LuPSMA PRLT therapy for progressive and metastatic CRPC prostate cancer. It's personalized medicine at its best: "See it, then zap it."
<div class="ivm-view-attr__img-wrapper
">
Two Aspects of Nuclear Medicine
Targeted therapy: Radioactive medicines deliver treatment directly to cancer cells while sparing most healthy tissue.
Used inthyroid cancer, prostate cancer, lymphomas, and neuroendocrine tumors.
Think of it as asmart bombprecise and powerful.
<div class="ivm-view-attr__img-wrapper
">
Past, Present and Future of Theranostics
Clinical impact:
Lu-177 PSMA therapy has shown 40% reduction in risk of death in advanced prostate cancer (VISION trial).
NETTER-1 trial showed 79% progression-free survival improvement with Lu-177 DOTATATE in neuroendocrine tumors.
In India, more than 10,000 Lu-177 therapies have been performed in the last 5 years, positioning the country as a regional hub for theranostics.
The Numbers Nuclear Medicine Worldwide : Nuclear medicine is booming, driven by aging populations and cancer rises.
Diagnostic Nuclear Medicine: ~40 million (4 crore) procedures annually worldwide.
USA: ~20 million scans/year.
India: ~0.51 million scans/year (rapidly growing).
Therapeutic Nuclear Medicine:
In 2023,over 100,000 theranostic procedures were performed globally, with rapid growth in prostate and neuroendocrine treatments.
Growing at1015% annually worldwide.
Theranostics centers expanding rapidly, especially in prostate and neuroendocrine cancer treatment.
Market value:
$10.19B in 2024, projected to $42B by 2032 (CAGR ~19%). Theranostics segment growing fastest at 13-15% CAGR.
USA: $5.12B in 2023, to $16.85B by 2033 (CAGR 12.6%). High adoption of PET-CT (over 2,000 scanners).
India: Rapid growth with 300+ centers. ~1-2 million procedures/year. Market to $1.07B by 2030 (CAGR 10.9%), fueled by affordable tech and rising cancer cases (1.4M new/year).
Therapeutic procedures (like radioiodine therapy) grew 20% globally post-COVID, with India seeing 25% annual increase due to better access.
Future: Bright and Innovative
By 2030, theranostics could dominate cancer care. Prospects include:
Expansion to Lung, breast, brain, pancreatic, and ovarian cancers.
Alpha emitters (like Ac-225) for resistant cancers.
AI-integrated imaging for smarter imaging interpretation, predictive response modeling and faster, more accurate targeting.
Combination with immunotherapy.
ImmunoPET tracers: tracking immune checkpoint inhibitors in real time
Personalized dosimetry: tailoring therapy dose to maximize tumor kill minimize toxicity
Challenges: Supply chain for rare isotopes, but advancements in cyclotron production are helping.
Myths vs Facts
Myth: Nuclear medicine is unsafe because of radiation. ✅ Fact: Radiation doses are carefully regulated, often equal to or less than a CT scan. It is safe, regulated, and used worldwide for decades. Doses are low a PET scan is like 2-3 years of natural background radiation. Tracers decay fast (hours/days), and benefits outweigh risks.
Myth: The radioactivity stays in your body forever.✅Fact: Most tracers are eliminated naturally within days. No long-term buildup.Myth: It's painful or invasive.✅Fact: Just an IV injection or pillless invasive than surgery, no pain beyond a needle prick.
Myth: It causes cancer✅Fact: Risk is minimal (1 in 10,000 for diagnostics). It's used to fight cancer, not cause it.Myth: Only for end-stage cancer.✅Fact: Great for early detection and monitoring, improving survival rates by 20-30% in many cases.Myth: PET-CT replaces all other scans. ✅ Fact: Different scans answer different questions. PET-CT complements, not replaces.
Myth: Once treated with radiotheranostics, you glow or are radioactive. ✅ Fact: The radiation is medical-grade, controlled, and leaves the body safely.
Why It Matters for PatientsFor patients, nuclear medicine means:
Less invasive testsMore confidence in diagnosis
Tailored Personalised treatmentBetter chances of cure and survival
Take-Home Messages
Nuclear medicine is not science fictionitsscience saving lives daily.
It allows doctors tosee cancer earlier, track it better, and treat it smarter.
FromHevesys tracer principle to modern PET-CT and theranostics, the field has grown into a cornerstone of cancer care.
India is catching up fast with global trends, and access is expanding.
The future promisespersonalized, precise cancer care with nuclear medicine at its heart.
Bust the myths: It's safe, precise, and life-saving.
Nuclear medicine is like a torchlight in the dark tunnel of cancerhelping doctors see clearly, act precisely, and give patients hope.
Nuclear medicine is not just scienceits a blend of technology and compassion, guiding modern cancer care.
Next time you hear about PET scans or targeted radiotherapy, remembertheyre part of a powerful field called nuclear medicine that combines vision with cure.
Have you or your loved one ever had a PET-CT or heard about nuclear medicine therapy? Share your thoughts in the comments.By Dr. Aashish Gambhir, Director Head, Nuclear Medicine
Andromeda Cancer Hospital

The (Rising) Burden of Cancer What is causing this silent epidemic?
Cancer is one of the leading non-communicable diseases globally. Further, the burden of cancer is rising continuously. While significant progress has happened in early diagnosis and treatment of cancer, the burden of death due to cancer is still high.
Cancer is the 2nd leading cause of death worldwide and causes nearly 15 to 16% of all deaths.
In India too, cancer is a major contributor to the causes of death, 2nd overall in urban areas and 4th overall in rural areas.
The cliched saying is A stitch in time saves nine but it may be highly relevant when it comes to cancer. Preventing is cancer is theoretically much easier than treating a cancer. However, each and every member of the society needs to be aware of the causes of cancer.
Individual action and commitment plays an important role in cancer prevention. Government policies and programs are important but can not work in isolation without public participation.
With this background, we are sharing the list of major causes of cancer globally and also highlight their importance based on gender.
Recognized Causes and Risk Factors for Human Cancer
A. Lifestyle-related
Tobacco use (smoked and smokeless)
Alcohol consumption
Dietary factors: processed/red meat, low fruits/vegetables, obesity-promoting diets
Obesity / overweight
Physical inactivity
B. Environmental and occupational
Air pollution (PM2.5, diesel exhaust, indoor coal smoke)
Occupational exposures: asbestos, silica, benzene, formaldehyde, wood dust, vinyl chloride, certain metals (arsenic, cadmium, chromium, nickel)
Radiation: ionizing radiation (X-rays, gamma rays, radon), ultraviolet radiation (sunlight, tanning beds)
C. Biological / infectious agents
Viruses: HPV, HBV, HCV, EBV, HTLV-1, KSHV, MCPyV
Bacteria: Helicobacter pylori
Parasites: Opisthorchis viverrini, Clonorchis sinensis, Schistosoma haematobium
D. Hormonal and reproductive
Endogenous hormones: prolonged estrogen exposure (early menarche, late menopause, nulliparity, hormone replacement)
Exogenous hormones/medications: oral contraceptives, menopausal hormone therapy, DES
Immunosuppressive drugs (tacrolimus, azathioprine)
E. Genetic and host-related
Inherited cancer syndromes (e.g., BRCA1/2, Lynch, Li-Fraumeni)
Family history (polygenic risk)
Ageing (strongest single risk factor, reflects cumulative mutations and immune decline)
Cancer Risk Factors Grouped by Gender
Predominantly male cancers / risks
Tobacco use (still higher in men globally lung, head neck, bladder, esophagus, pancreas)
Occupational exposures (asbestos, silica, diesel exhaust, metals historically more in men)
Alcohol use (higher consumption rates in men)
HPV oropharyngeal cancer risk increasingly seen in men
<div class="ivm-view-attr__img-wrapper
">
Predominantly female cancers / risks
Reproductive/hormonal factors (estrogen/progesterone exposure breast, endometrial, ovarian)
HPV cervical cancer (exclusively female)
Hormone therapy (HRT, OCPs, DES)
<div class="ivm-view-attr__img-wrapper
">
Both men and women
Tobacco, alcohol, obesity, diet, physical inactivity
Air pollution, radiation, environmental exposures
Infectious causes: HBV/HCV (liver), EBV (lymphoma/nasopharynx), H. pylori (stomach), parasites
Genetic predispositions
<div class="ivm-view-attr__img-wrapper
">
Relative Contribution to Cancer Burden (Attributable Fraction)
Global estimates (WHO / IARC / GLOBOCAN; varies by region):
Tobacco ~22% of all cancer deaths worldwide (8 million deaths annually). Causes lung, head neck, bladder, esophagus, pancreas, stomach, kidney.
Infections ~1315% of cancers globally, higher in developing countries. HPV, HBV, HCV, H. pylori dominate.
Alcohol ~56% of cancers worldwide. Strong links: oral cavity, pharynx, larynx, esophagus, liver, breast, colorectum.
Obesity/overweight physical inactivity ~58% globally. Strong for breast (postmenopausal), colorectal, endometrial, pancreas, kidney, liver.
Diet (low fruits/vegetables, high processed meat, low fiber, high salt) ~5%.
Occupational exposures ~35% of cancers (but higher in men in industrialized areas).
Air pollution (ambient + household) ~23% globally; larger share in Asia.
Radiation (ionizing + UV) ~2%. UV is major for skin cancers (melanoma, squamous, basal).
Genetic predisposition ~510% of cancers due to inherited mutations.
Ranking by Estimated Impact (Global cancer burden)
Tobacco (22%)
Infections (1315%)
Alcohol (56%)
Obesity / overweight / inactivity (58%)
Dietary factors (5%)
Occupational exposures (35%)
Air pollution (23%)
Radiation (2%)
Genetic predisposition (510% but varies by cancer type)
<div class="ivm-view-attr__img-wrapper
">
Key Takeaways:
The largest preventable causes globally are tobacco, infections, alcohol, and obesity-related factors.
Men: Major risk related to tobacco, alcohol, and occupational risk factors.
Women: Major risk related to reproductive/hormonal factors, HPV, obesity-linked cancers.
Both sexes: Diet, infections, air pollution, and radiation.

The Challenges of Breast Cancer in IndiaIntroduction
Breast cancer is the commonest cancer of women globally and in India. According to GLOBOCAN 2022, breast cancer accounts for nearly 23 lakh new cases each year. In India, nearly 2 lakh cases of breast cancer are diagnosed every year. In India, 1 in 28 women will develop breast cancer during their lifetime.
Due to intense research efforts, breast cancer has become highly curable. Unfortunately, 5060% of breast cancer cases in India are still diagnosed at Stage 3 or beyond, compared to less than 10- 20% in high-income countries. Five-year survival rate in western countries is nearly 85 to 90%. However, India lags behind significantly in this regard: the five-year survival figure is around 60% only in India. The poor outcomes of breast cancer treatment in India, as compared to developed countries, stem from a combination of medical, social, infrastructural, and economic factors which can be broadly grouped into two as patient factors and system factors.Reasons for Worse Survival of Breast Cancer Patients in India
Late diagnosis of breast cancer
Lack of access to standardized high quality breast cancer care for a large proportion of breast cancer patients
The problem of delay in diagnosis can be managed at the patient (society) level by spreading awareness, guiding them to early symptoms and signs of breast cancer, educating them about breast self-examination, encouraging them to seek medical help early.The problem of delay in diagnosis at the level of healthcare system needs multi-pronged efforts. It is important for the clinicians who see patients with breast symptoms to understand that early diagnosis and prompt initiation of treatment are critical for successful outcome of breast cancer treatment. Every clinician must know that for early and accurate diagnosis, the most reliable step is triple assessment clinical examination, imaging, and pathological confirmation of diagnosis by biopsy.
Clinical ExaminationA detailed history (age, family history, duration, changes in size) and systematic clinical examination of the breasts and axillae to detect lumps, skin changes, nipple retraction, discharge, or palpable nodes.
Imaging
Mammography: gold standard for women over 40
Ultrasound: especially useful for younger women with dense breasts
MRI: reserved for complex cases, multi-focal disease, or inconclusive findings.
Pathological Assessment
Image guided core needle biopsy, which gives not just diagnosis but tumour grade, receptor status (ER, PR, HER2), all this information is essential for treatment planning.
When all three correlate, the diagnostic accuracy exceeds 99% minimizing false negatives and false positives.
<div class="ivm-view-attr__img-wrapper
">
Triple Assessment for Breast Cancer (Examination, Imaging, Biopsy)
Challenges in Breast Cancer Care in India
In this article, we would like to describe some of the challenges we perceive (as breast specialists) which define the breast cancer care in our country.
Lack of Trained Breast Specialists
Breast cancer care is now a recognized specialty in India, and many young doctors are taking it up. However, most women with breast symptoms first visit healthcare providers who are not trained in breast examination or cancer screening.
Many healthcare providers lack training in proper breast examination.
Triple assessment (clinical exam + imaging + biopsy) is not routinely followed.
Important signs like skin changes or nipple retraction are missed.
Breast lumps are often dismissed as
Breast pain is treated casually with Vitamin E or evening primrose oil without proper tests.
Advanced cancers are mistaken for mastitis/breast infection.
Breast cancers during pregnancy are mostly misdiagnosed as pregnancy associated breast changes.
Many doctors manage breast cancers themselves despite not having the right skills.
This casual approach, poor clinical examination skills, lack of awareness about triple assessment (clinical exam, imaging, and biopsy), and wrong interpretation of symptoms lead to diagnostic delays and worse outcomes. It is necessary to train and sensitize healthcare providers to detect and refer suspected cases early.
Problems in Breast Imaging and ReportingStandards of breast imaging in India vary widely, especially between urban and rural centres.
Many reports dont follow BIRADS guidelines or assign wrong categories.
Indian women have denser breasts, making mammograms harder to interpret.
Poor communication between doctors and radiologists, outdated machines, and shortage of trained breast radiologists add to the problem.
In rural areas, lack of modern imaging and expert reporting means more women are diagnosed late.
Younger women (more likely to have triple-negative cancers) often get benign-sounding reports, leading to missed diagnoses.
Continued Use of FNAC (Fine Needle Aspiration Cytology) for diagnosis and planning treatmentAlthough once common, FNAC has major drawbacks:
Less accurate than core needle biopsy (more false negatives).
Does not give enough tissue for ER/PR/Her2/Ki67 testing.
Cannot confirm invasion or grade the tumour.
No information on margins, lymphovascular invasion, or surrounding tissue.
Sampling errors are common, leading to missed cancers.
Overuse of Direct Excision Biopsies without Proper AssessmentMany women are advised to have their lump removed directly without imaging or core biopsy because breast surgery is wrongly thought to be easy.
Myths about biopsy spreading cancer make patients agree to excision.
Sometimes the removed lump is not sent for pathology, denying diagnosis.
Specimens are often removed into pieces or not oriented for margins, making accurate size and margin clearance assessment impossible.
Poorly placed surgical incisions may prevent breast conservation later.
Axillary node assessment is not done, requiring another surgery.
These issues can delay treatment and reduce chances of cure.
Starting Treatment without knowing IHC Results (Biological profile of the individuals breast cancer)Today, breast cancer treatment is personalized. Information on tumour biology (ER/PR/Her2/Ki67) and stage is essential for planning.
Patients with certain subtypes (triple-negative, Her2-positive) or large tumours benefit from neoadjuvant systemic therapy before surgery.
Without these details, outdated mastectomy first approaches deny patients the advantages of modern treatment.
Overuse of MastectomyMany women undergo mastectomy solely based on FNAC results, sometimes even when its a false-positive.
Breast conservation surgery (BCS) is equally safe for early cancers, but lack of surgeon training, old beliefs, and ignorance mean that 2/3 of breast cancer surgeries in India are still mastectomies.
Mastectomy can cause lifelong psychological distress. Although, breast reconstruction can be safely performed after mastectomy, it requires advanced surgical skills, extra days of hospitalization and post-surgical recovery and significantly additional cost compared to the breast conservation surgery.
Incomplete MastectomiesWhen performed by untrained surgeons, mastectomy may leave significant breast tissue or lymph nodes behind.
This leads to high recurrence risk and rapid disease progression.
Completion surgery is technically difficult and may prevent breast reconstruction.
Scarring and poor incision placement complicate future treatments.
Delays in adjuvant therapy worsen prognosis.
Very limited availability of resources and skills for sentinel node biopsyWithout SLNB, many women with early breast cancer undergo full axillary lymph node dissection, even when nodes are not involved.
This causes high risk of complications such as arm swelling (lymphedema), shoulder stiffness, numbness, chronic pain, and higher risk of wound problems.
It prolongs recovery, lowers quality of life, and leaves lasting disability.
SLNB is a safer, less invasive, guideline-recommended procedure.
Many Indian patients face avoidable harm and overtreatment due to this.
Issues related to systemic Therapy for Breast Cancer
Drug availability cost barriers: Newer targeted drugs and supportive medicines may be unaffordable or unavailable for many patients.
Incomplete biological profiling: ER, PR, Her2, and Ki67 tests are sometimes not done before starting chemotherapy, leading to non-personalized treatment.
Use of outdated regimens: In smaller centres, older protocols may still be used instead of evidence-based modern regimens.
Risk of errors high toxicity: Incorrect dosing, poor monitoring, and lack of proper supportive care can cause preventable side effects.
Infrastructure limitations: Many places lack dedicated day-care chemotherapy units, proper infection control, and trained oncology nurses.
Inadequate patient counselling: Patients may not receive enough guidance on side effects, fertility preservation, or the importance of completing all cycles.
Poor adherence to treatment: Due to side effects, cost, or lack of awareness, some patients discontinue therapy early, reducing chances of cure.
Issues related to Radiotherapy for Breast CancerLimited availability of advanced technology: Many centres still use outdated cobalt machines instead of modern linear accelerators with 3DCRT, IMRT, or IGRT capabilities.
Geographic and access barriers: Radiotherapy facilities are concentrated in larger cities, forcing rural patients to travel long distances daily for several weeks.
Long waiting times: High patient load and limited machines lead to delays in starting treatment, which can worsen outcomes.
Lack of breast-specific techniques: Inadequate use of advanced methods like deep inspiration breath-hold (DIBH) to protect the heart and lungs in left-sided cancers.
Inconsistent treatment quality: Variation in contouring, planning, and dose delivery between centres due to lack of standard protocols or quality audits.
Shortage of trained staff: Limited numbers of radiation oncologists, physicists, and technologists with specialized breast cancer training.
Side effect management gaps: Insufficient counselling and follow-up for managing skin reactions, fatigue, lymphedema, and late toxicities.
Financial burden: High cost of advanced radiotherapy techniques and travel/accommodation expenses during prolonged treatment courses.
Challenges in Genetic Counselling and Testing for Breast Cancer patients.Low awareness: Many patients and even healthcare providers are unaware of the role of BRCA and other genetic mutations in breast cancer risk.
Limited availability of trained counsellors: Very few centres have qualified genetic counsellors to guide patients and families.
High cost of testing: Genetic tests are expensive, often not covered by insurance, and unaffordable for many patients.
Access barriers: Testing facilities are concentrated in urban centres, limiting availability for rural populations.
Cultural and social stigma: Fear of discrimination, marriage-related concerns, and family pressure discourage many from testing.
Poor integration into routine care: Genetic risk assessment is not consistently incorporated into breast cancer evaluation and follow-up.
Lack of pre- and post-test counselling: Inadequate explanation of test implications can lead to anxiety, misinterpretation, or misuse of results.
Limited cascade testing: Family members at risk are rarely offered or encouraged to undergo testing.
Data privacy concerns: Fear of genetic information misuse due to weak legal safeguards.
Missed prevention opportunities: Without testing, high-risk women lose the chance for preventive measures such as enhanced screening, chemoprevention, or prophylactic surgery.
Concerns Related to Recurrence Score Testing in Breast CancerHigh cost: These tests are expensive and often not covered by insurance, making them unaffordable for many patients.
Limited availability in India:Most samples are sent abroad, increasing cost and turnaround time.
Delay in treatment decisions: Waiting for results can postpone the start of adjuvant therapy.
Lack of awareness: Many oncologists and patients are unfamiliar with the availability, benefits, and limitations of these tests.
Unclear applicability in all populations: Most validation studies are from Western populations; Indian-specific outcome data is limited.
Infrastructure and logistics: Sample collection, preservation, and international shipping may be challenging, especially in smaller centres.
Patient anxiety: Misunderstanding the meaning of recurrence scores can cause unnecessary worry or false reassurance.
Limited use in public sector: Government hospitals rarely offer or recommend such tests due to cost constraints.
Ethical and equity concerns: Only wealthier patients can access these tools, creating disparities in personalized cancer care.
In summary: Improving breast cancer outcomes in India requires:
Training doctors in proper clinical and diagnostic pathways.
Enforcing quality standards in imaging and reporting.
Phasing out FNAC for diagnosis in favour of core biopsy.
Working towards establishing early diagnosis and prompt initiation of treatment.
Making breast cancer treatment available and affordable to all the patients
Avoiding unnecessary or poorly performed surgeries.
Multi-disciplinary approach to ensure that the treatment decisions are made after full pathological and biological assessment.
Ensuring oncological safety and working towards preserving long term quality of life of the survivors.
<div class="ivm-view-attr__img-wrapper
">
Multi-disciplinary Board Meeting

Obesity and Cancer — What Everyone Should Know?(Eat and exercise your way to a healthy life.)
Welcome from Andromeda Cancer Hospital
At Andromeda Cancer Hospital, our mission is to bring useful health information straight to our community. Today, we tackle an important, often misunderstood topic: the connection between obesity and cancer. With obesity rates rising and cancer cases growing in Haryana and across India, its the right time to learn and take charge of our health.
Obesity is assessed by BMI but normal weight obesity also occurs and truncal fat is more harmful.
What Causes ObesityObesity develops from a mix of different factors working together. While eating more calories than the body burns is a major cause, theres much more to it. Here are some of the key contributors:
Unhealthy diet and overeating: Consistently eating more calories than needed, especially from high-sugar and processed foods.
Lack of physical activity: Sedentary lifestyles make it easier to gain weight.
Genetics: Family history can influence how your body stores fat and uses energy.
Hormonal imbalances: Conditions like hypothyroidism or changes in hormones can affect weight.
Medical conditions and medications: Some illnesses and medicines can contribute to weight gain.
Emotional stress: Stress or emotional eating may lead to consuming excess calories.
Environmental and lifestyle factors: Easy access to unhealthy foods, busy routines, and lack of resources for exercise also play a part.
Obesity has multiple underlying causes
In summary, obesity is usually the result of a complex interplay between lifestyle habits, biology, medical issues, and our environment.
Quick Facts:
The burden of obesity is rising all over the world.
In India, over 135 million people are affected by obesity. The burden has increase by nearly 200% in last two decades and is expected to double in the next 10 to 15 years.
How Are Obesity and Cancer Connected?Many people dont realize that carrying extra weight can actually increase ones risk of developing cancer. Research has shown that obesity is a risk factor for several types of cancers. WHO and IARC have identified 13 different cancers whose risk is increased by obesity. These include.
Uterine (endometrial) cancer
Breast cancer (especially after menopause)
Ovarian Cancer
Cancer of oesophagus
Colon and rectum cancer
Liver cancer
Gallbladder cancer
Pancreatic cancer
Stomach cancer
Kidney cancer
Multiple myeloma
Thyroid cancer
Meningioma
See the Connection Between Obesity and Cancer:Why Does Obesity Increase Cancer Risk?
You dont need to be a scientist to understand the basic idea: Think of your body like a car engine. If its overloaded and running too hot, problems will follow.
Extra Fat Increases Inflammation: Chronic, low-level inflammation caused by obesity can damage cells and lead to cancer.
Growth factors: Excess fat in the body leads to insulin resistance and increases the release of insulin and related growth factors in response to food. These growth factors increase the cancer risk.
Hormone Changes: Excess fat converts some hormone released from the adrenals to active oestrogens and the higher levels of postmenopausal oestrogens are associated with higher risk of cancers such as breast cancer, endometrial cancer, etc.
Impact on Immunity: Obesity can weaken your immune system, making it harder to detect and fight off cancer cells.
What Can You Do? Actionable TipsYou have more power than you think! Start with small, lasting changes:
Choose local, seasonal fruits and vegetables. Try to add millet (bajra, jowar) and pulses to your meals. Limit processed foods and sugary drinks.
Move more every day. Even a 30-minute walk in your neighbourhood or some light yoga can make a big difference.
Exercising is important for obesity control. Diet alone will not be sufficient in the long run.
Regular health checkups. Early detection saves lives! Dont hesitate to consult our team for advice.
Family support.Involve your loved ones in healthy habits together, its easier.
What the other harms in cancer patients who are obese?The problems dont stop at increasing the risk of cancer. Cancer treatment is more problematic in obese patients, complications of treatment are higher, and the treatment may be less effective. Obese patients may have higher risk of cancer recurrence.
Is it possible to get out of this vicious cycle?Yes, if you can control your weight, you can reduce your risk of cancer. Reduction in weight after cancer diagnosis and treatment also reduces the side effects of cancer treatment, improves long term survival and improves quality of life.Various approaches including exercise, dietary modifications, anti-obesity medications, bariatric surgery can all be used depending upon the individual patients needs and choices.
At Andromeda Cancer Hospital, we offer nutrition guidance, tailored cancer screenings, and weight management support. Our doors are always open for you and your family.
QA Busting Common MythsLets clear up some common misunderstandings:Myth 1: Only junk food makes you obese.Reality: While diet matters, other factors like low physical activity, genetics, and even stress play important roles.Myth 2: Thin people dont get cancer, and overweight people always do.Reality: Anyone can get cancer but being obese increases risk for certain cancers. Maintaining a healthy weight lowers (but doesnt eliminate) your risk.Do you have questions or want to share your story? Wed love to hear from you. Write to us or visit Andromeda Cancer Hospital lets fight cancer together.
For free health talks, screenings, and support, contact us at 9138111625.
Catch our next newsletter in your LinkedIn feed! Subscribe to the newsletter.
Stay informed. Follow Andromeda Cancer Hospital here on LinkedIn for trusted tips, inspiring stories, and upcoming events.Have a question or a topic youd like us to cover? Comment below or message us directly!
Together, lets build a healthier India, healthier Haryana and healthier Sonipat. Let us fight cancer at all levels, prevent it, diagnose it early, treat it better and help patients live a happy and healthy cancer free life.
Please visit our YouTube channel for videos on Obesity and Cancer.
You can also visit and subscribe to the YouTube channel for regular informative videos about cancer awareness.

Immune Therapy in Cancer: mRNA Vaccines.....What is mRNA?
mRNA (messenger RNA or ribonucleic acid) is polymeric molecule produced in the cells by a process of transcription using DNA. mRNA is a template used for synthesis of proteins. The proteins then get modified in various ways and serve their diverse functions. Some of the proteins serve as antigens that are recognized by immune cells of the body and body can mount an immune defence against these antigens.
What are mRNA vaccines and how do they work?
An mRNA vaccine is a revolutionary type of immunization that uses mRNA prepared in the laboratory and injected. It instructs the body cells to produce a viral or tumor-related protein. This stimulates the immune system to build both antibody (humoral) and T-cell (cellular) responses.Steps in the process for mRNA vaccine preparation and usage
Antigens have to be identified from the viral pathogens or cancer cells
mRNA are produced in the laboratory using equipment that can generate mRNA molecules in the instructed sequence.
The mRNA molecules are packaged in lipid nanoparticles (LNPs).
The vaccine is administered via intramuscular injection.
Cells translate the mRNA to produce the target antigen (e.g., SARS‑CoV‑2 spike protein).
When the antigen appears on the cell surface, the immune system recognizes it as foreign and activates adaptive immunity.
The mRNA is degraded afterward (and does mRNA vaccines across different diseases.
Infectious diseases: Viral Targets: COVID‑19 mRNA vaccines (PfizerBioNTech Comirnaty Moderna Spikevax) are the only currently authorized mRNA vaccines.
There are many others in development/testing: influenza, RSV, CMV, EBV, Zika, HIV, norovirus, Hepatitis C, genital herpes, malariamany already in Phase 13 clinical trials
Bacterial targets: A novel candidate vaccine against Yersinia pestis (plague) recently showed 100% efficacy in mice; human trials still pending.How many mRNA vaccines are approved?
COVID‑19: PfizerBioNTech Comirnaty Moderna Spikevax These are the only licensed mRNA vaccines worldwide to date
No mRNA vaccines have yet received approval for other diseases; many are in varying stages of clinical trials.
mRNA vaccines in cancer: current landscape
Oncology research in mRNA vaccines is rapidly advancing:
Clinical trial status: Over 120 clinical trials are exploring mRNA vaccines for lung, breast, prostate, melanoma, pancreatic, brain cancers and more.
Safety tolerability: Multiple trials have found mRNA cancer vaccines to be well tolerated with manageable side-effects, sometimes less than traditional chemotherapy.
Promising efficacy signals: A Phase I personalized neoantigen mRNA vaccine for pancreatic cancer (16 patients) generated specific T-cell responses; responders remained recurrence-free for up to 18 months.
Personalized vaccine platforms (e.g., Autogene cevumeran) are entering Phase II trials
Moderna/Mercks mRNA‑4157/V940 combined with pembrolizumab showed a 44% reduction in melanoma recurrence in Phase II, now progressing into Phase III.
"Universal" vaccine approach: Preclinical studies in mice (University of Florida) indicate a general mRNA booster that primes the immune system when used with checkpoint inhibitors, though human trials are still in planning. This particular vaccine could work against multiple different type of cancers and not be dependent on cancer specific antigens.
Looking Ahea
Challenges:The field of mRNA vaccines is relatively new. The vaccines against Covid received emergency use approval. Long term safety data is necessary. However, the concept underlying the mRNA vaccines is very promising. However, scientific research has to complete all phases of testing and show evidence of benefit with acceptable toxicity profile. Regulatory issues, cost of therapy, scaling personalized vaccine production, delivery methods, etc are issues to be handled.
Hopeful future: If ongoing trials confirm efficacy, mRNA vaccines could become vital in adjuvant cancer therapy to prevent recurrence and possibly treat ongoing cancers.
Conclusions
mRNA vaccines work by instructing cells to produce antigens that drive immune responseoffering rapid, flexible platforms.
Today, only COVID‑19 mRNA vaccines are licensed.
mRNA tech is in active trials for infectious diseases and is showing very encouraging progress in oncology, with multiple phase II/III human trials underway.
Several cancer-specific mRNA vaccines have successfully entered human trials, with early data indicating both safety and potential efficacy.

De-escalation of Treatment in Breast Cancer Balancing Cure and Quality of Life
Breast cancer treatment has transformed dramatically over the last five decades.It used to be one-size-fits-all approach. It was dominated by radical mastectomy/modified radical mastectomy in nearly all patients and chemotherapy and radiotherapy in selected cases.
Mammographic imaging is a game changer for screening and early diagnosis
It is now a highly personalized approach with individualized decision making. At the heart of this evolution lies a powerful idea: de-escalation of treatment.De-escalation means deliberately reducing the intensity or extent of surgery, chemotherapy, or radiotherapy without compromising cure. The aim is not only to save lives but also to preserve quality of life, minimize side effects, and avoid long-term harm.This represents a shift from maximum tolerated treatment to minimum effective treatment.
There are multiple approaches or modalities used in breast cancer treatment today.
Why De-escalation MattersSurvival rates have improved with earlier detection and better systemic therapies. But aggressive treatments often leave lasting scars:
Chronic lymphedema after axillary dissection
Disfigurement and trauma after mastectomy
Infertility and menopause from chemotherapy
Cardiotoxicity, fatigue, and secondary cancers after radiotherapy
These burdens have led oncologists worldwide to ask: Can we treat less and still cure? Increasingly, the answer is yeswhen patients are carefully selected.
Areas of De-escalation
1. Surgery
Breast Surgery Breast Conservation after NAST: In large operable and selected locally advanced cancers, neoadjuvant systemic therapy (NAST) often shrinks tumours, making breast conservation surgery (BCS) possible. With proper imaging, margin control, and radiotherapy, outcomes are comparable to mastectomy, with superior cosmetic and psychological benefits. Omission of Surgery: Trials are exploring whether patients achieving complete response after NAST can safely avoid surgery. While promising, this approach requires rigorous imaging, biopsy confirmation, and close follow-up in clinical trial settings before wider adoption.
Axillary Surgery Sentinel Lymph Node Biopsy (SLNB): SLNB has replaced axillary lymph node dissection (ALND) in node-negative patients, reducing lymphedema while providing accurate staging. Recurrence rates remain equivalent to ALND. Avoiding ALND in Limited Nodal Disease: Trials such as ACOSOG Z0011, IBCSG 23-01, AMAROS, SENOMAC, and SENODAR show that patients with minimal nodal disease on SLNB can avoid ALND, sometimes substituting axillary radiation. Omission of Axillary Surgery Altogether: The SOUND trial demonstrated that early breast cancer patients with negative axillary ultrasound can avoid even SLNB without compromising safety. This major advance reduces morbidity, shoulder dysfunction, and preserves body image.
2. Radiotherapy
Hypo-fractionated Schedules: Delivering higher doses in fewer sessions is now standard, offering equal efficacy, lower toxicity, and more convenience.
Partial Breast Irradiation: In very low-risk patients undergoing BCS, targeting only the tumour bed instead of the entire breast achieves safe outcomes.
Omission of Radiotherapy: In carefully chosen elderly, low-risk patients, omission does not compromise survival and spares them from toxicity.
3. Systemic Therapy
Genomic Assays: Tests like Oncotype DX and MammaPrint help identify hormone-receptor positive patients who can avoid chemotherapy, receiving only endocrine therapy.
Shorter Chemotherapy Courses: Selected regimens with reduced cycles show comparable results, minimizing cumulative toxicity.
Targeted Therapy: For HER2-positive disease, studies suggest that shorter durations of trastuzumab may suffice, lowering cardiotoxicity risk.
Various approaches are combined based on finding the disease biology through molecular testing
4. Endocrine TherapyEndocrine therapy improves survival but prolonged use causes menopausal symptoms, bone loss, and adherence problems. Evidence indicates that in some low-risk patients, five years of therapy may be adequate compared to ten years.Risks of De-escalationDe-escalation carries challenges that must be weighed carefully:
Undertreatment: Lower-intensity therapy may increase recurrence in some patients.
Tumour Heterogeneity: Low-risk appearance does not always equal indolent biology
Compensatory Overtreatment: Less surgery is often offset by more systemic therapy or radiation, shifting rather than reducing toxicity.
Psychological Concerns: Some patients equate less treatment with less cure, creating anxiety.
Limited Long-Term Data: Many de-escalation trials have short follow-ups; survival data over decades are awaited.Thus, de-escalation must be evidence-driven, guided by tumour boards, and aligned with patient preferences.
Benefits of De-escalationDespite the risks, the advantages are substantial:
Reduced Toxicity: Lower rates of lymphedema, cardiotoxicity, infertility, and secondary malignancies.
Improved Quality of Life: Better cosmetic outcomes, body image, and emotional recovery.
Cost Savings: Particularly valuable in low-resource settings like India.
Faster Recovery: Enables earlier return to family, work, and normal life.
Patient-Centred Care: Aligns treatment with individual biology and personal values.
Long-Term Advantages
Personalized Medicine: Molecular profiling and AI tools will refine risk stratification, allowing precise tailoring of therapy intensity.
Healthcare Sustainability: Avoiding overtreatment conserves resources, improving access to effective care.
Survivorship Focus: With rising survival rates, quality of life and long-term well-being take centre stage. De-escalation prevents chronic complications, ensuring survivors thrive beyond cancer.
Global Relevance: In resource-limited countries, evidence-based de-escalation provides safe, affordable care without compromising outcomes.
The Way ForwardDe-escalation is not a universal formula. Success requires:
Careful patient selection using clinical, pathological, and molecular tools
Multidisciplinary tumour board decision-making
Shared decision-making, incorporating patient preferences
Robust clinical trial participation and long-term data generation
Ultimately, de-escalation reflects the art of modern oncology: treating smarter, not harder.
ConclusionBreast cancer care has entered an era of precision and compassion. De-escalationacross surgery, radiotherapy, and systemic therapybrings together the twin goals of cure and quality of life.For patients, it means fewer scars and a fuller life. For oncologists, it represents evidence-based, humane medicine. For society, it ensures sustainable, affordable cancer care.The challenge now is to refine our tools and judgment so that every woman receives not just the best chance of survival, but the best chance of living well.Andromeda Cancer Hospital now offers world class cancer treatment to patients of Haryana, Delhi and Northern India. For breast cancer management, it has a specialized centre of excellence "Andromeda Breast Cancer Centre".Andromeda Cancer Hospital Youtube Channel: http://www.youtube.com/@andromedacancerhospitalYou will find a treasure trove of informational videos at this channel. Visit this page and find out for yourself. Also request you to follow and share the link to the youtube channel.
